Neutrophil-derived vesicles control complement activation to facilitate inflammation resolution
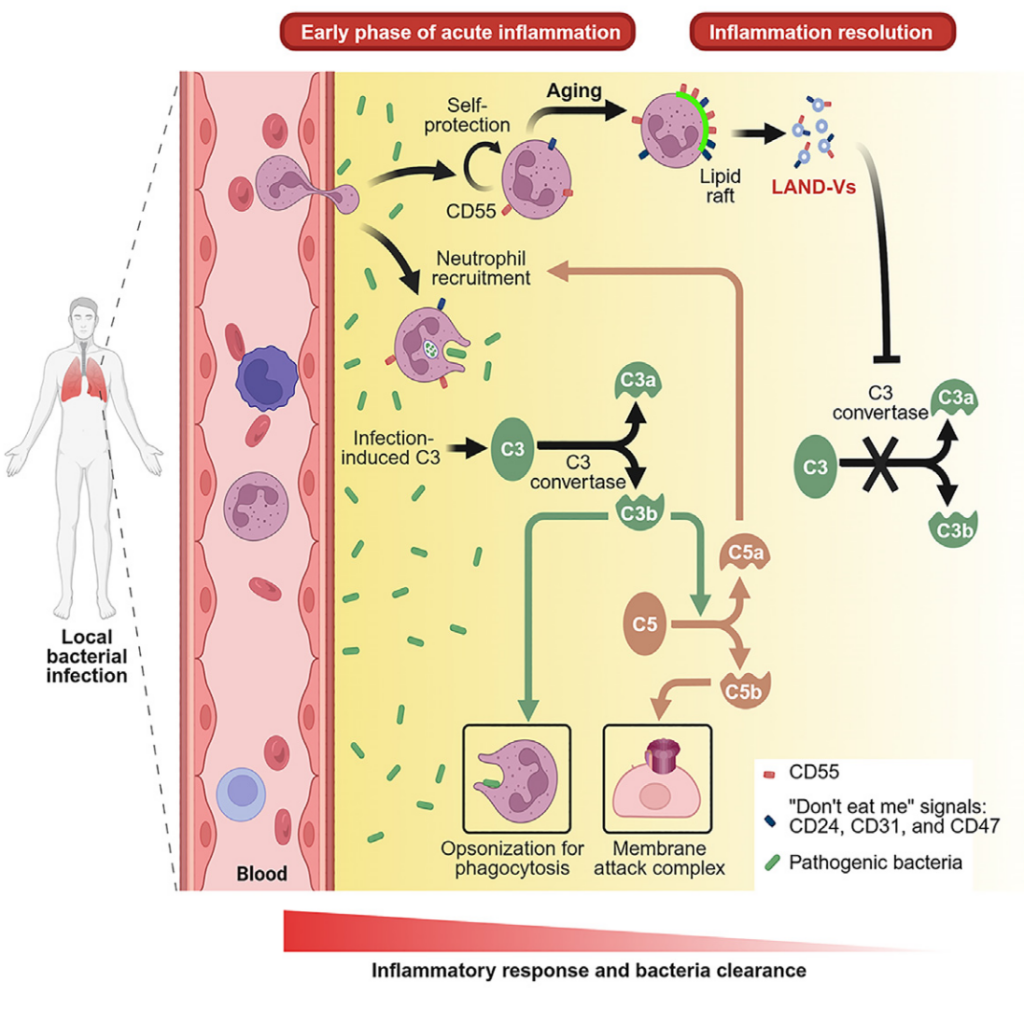
Neutrophils are short lived innate leukocytes involved in the early response to infection, and a major cellular player in the onset of acute inflammatory response. While the functions of live neutrophils is well understood, their contribution to immunity carries-on beyond their life span, through the production of extracellular traps (NETs), and the release of apoptotic bodies (ApoBDs). In a recent publication titled “Neutrophil-derived vesicles control complement activation to facilitate inflammation resolution”, Hsu et al. characterized a new type of vesicles released from human and mouse neutrophils during cellular aging. These vesicles were coined “LAND-Vs”, for “large aging neutrophil-derived vesicles”, and were generated by mechanisms different from neutrophil-derived ApoBDs. LAND-Vs acted as inhibitors of the inflammatory response. Specifically, LAND-Vs surface was enriched in the complement factor H and the complement decay-accelerating factor (DAF, CD55). Both factors inhibited the activation of the central C3 convertase, thus preventing excessive complement system activation and subsequent tissue damage. LAND-Vs were also uniquely expressing high levels of “do not eat me” signals, including CD47, preventing their rapid disappearance. In vivo models demonstrated that blocking LAND-Vs complement inhibition impaired the resolution of inflammation, highlighting their crucial role in immune homeostasis. By promoting a virtuous negative feedback loop, LAND-Vs enabled the transition from a pro-inflammatory to a resolution phase, effectively limiting neutrophil accumulation and facilitating the clearance of apoptotic cells.
Hsu et al., Neutrophil-derived vesicles control complement activation to facilitate inflammation resolution, Cell (2025), https://doi.org/10.1016/j.cell.2025.01.021
Research Highlight in Nature Immunology
Fabien Loison
Co-author

