Immunogenicity of five Encapsidated DENV antigens supports their potential as a safe and protective subunit vaccine candidate
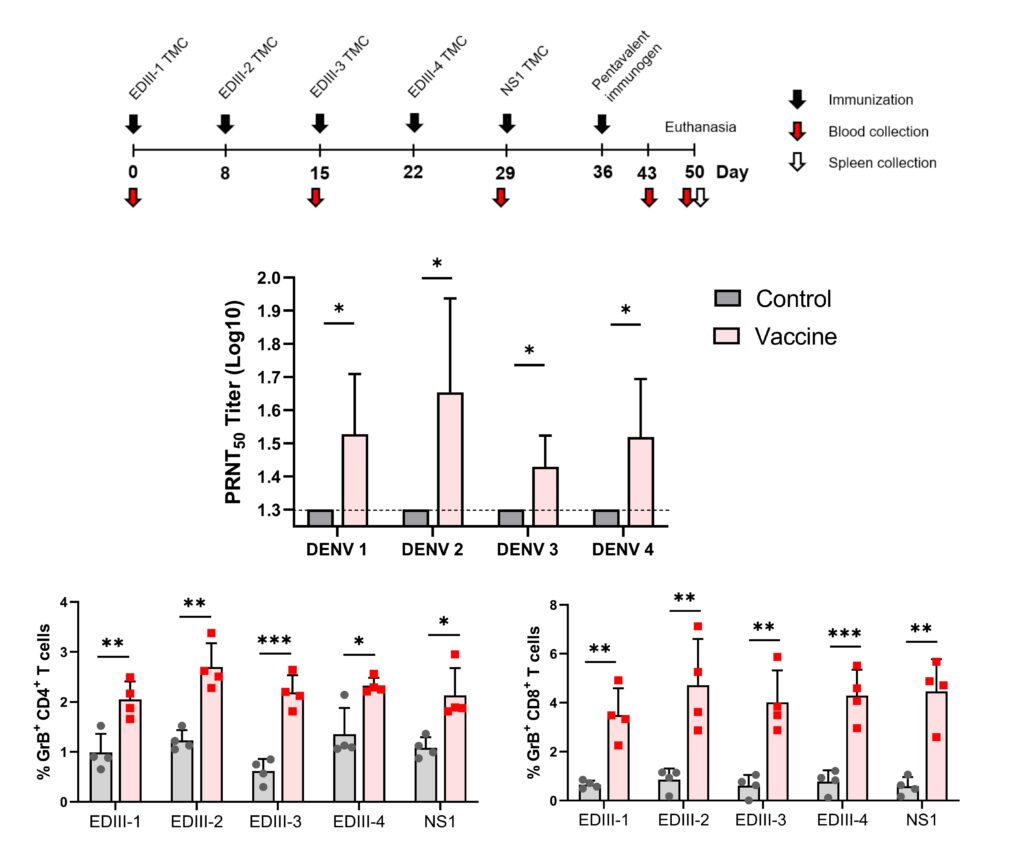
Dengue virus infection continues to pose a major global health challenge, affecting approximately 3.9 billion individuals annually. Unfortunately, the efficacy and safety profiles of currently licensed dengue vaccines remain debatable. This highlights the need for a next-generation vaccine that provides protection against all DENV serotypes. In this study, the immunogenicity of five DENV proteins was evaluated, including the envelope domain III proteins of all four dengue virus serotypes and C-terminally truncated NS1 protein of DENV-2, loaded into N, N, N-trimethyl chitosan nanoparticles. We demonstrated, here, that sequential immunization with these encapsidated immunogens elicited antibody responses known to correlate with protection against all four DENV serotypes. These responses facilitated the elimination of both infected cells and virus particles through a multifaceted mechanism. Moreover, the immunization induced functional cytotoxic T cell responses to all tested immunogens. These findings indicate that immunization with the designed immunogens induces robust and protective immune responses against all four DENV serotypes. This form of immunogens offers a promising, safe and effective subunit dengue vaccine candidate.
Corresponding author: Sukathida Ubol
First-author: Mathurin Seesen

